Rhenium and Molybdenum complexes as catalysts in oxygen atom transfer reactions
Homogeneous catalysis with Mo and Re
Metal-catalyzed oxygen atom transfer reactions like epoxidations constitute a key step in the mechanism of organic oxidations and find broad applications in industry.
In the past years, rhenium has - alongside with molybdenum - proven to be a suitable metal for the development of oxidation catalysts, especially rhenium in the oxidation state +VII and, to a much smaller extent, in the oxidation state +V. Mechanistic aspects of rhenium(V) systems are largely unexplored up to now. The catalytic reduction of small oxo anions like ClO4- under mild conditions is a special property of oxorhenium(V) complexes, which we currently investigate.
Fig. 1: General schemes of epoxidation- and reduction catalysis.
Epoxidation catalysis
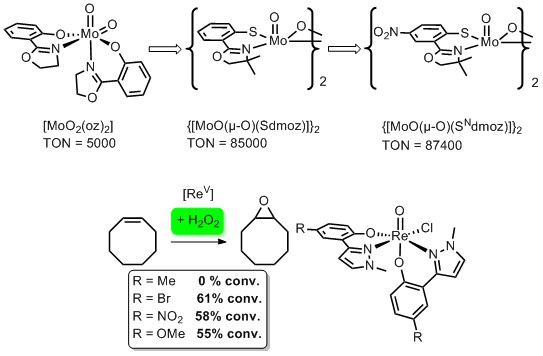
Over the last years we could significantly improve the catalytic activities in the epoxidation of cyclooctene of our Mo-catalysts. Coordinative unsaturation of the metal center as well as hemi-labile ligands seem to play a key role. In our Re chemistry we synthesized complexes that showed catalytic activity with the green oxidant H2O2. The substituents on the phenol ring seem to play a vital role here.
Fig. 2: Epoxidation catalysts with Mo und Re.
Catalytic reduction of oxyanions
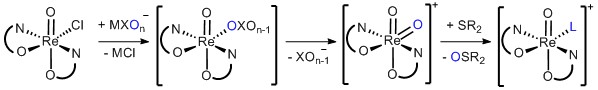
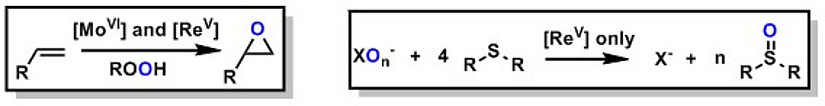
Certain oxorhenium(V) complexes are capable to reduce the kinetically stabile oxyanions perchlorate and nitrate under mild conditions. They operate via an oxygen atom transfer (OAT) mechanism, whereby the Re-center is alternating between the +V and +VII oxidation state. As acceptors, various sulfides can be employed, which in the last step of the cycle reduce the Rhenium back to oxidation state +V. Overall, the complete reduction of ClO4- to Cl- is possible via four sequential OAT steps. In contrast to perchlorate, nitrate has a much bigger ecological impact. Due to the excessive use of fertilizers in modern agriculture, too high nitrate levels in ground water are a widespread, global problem. Hence, a chemical catalytic reduction of nitrate to benign dinitrogen would be of great interest. The details of this fascinating reaction are currently investigated by us.
Fig. 3: Postulated reaction mechanism for perchlorate reduction.