Biomimetic Ligands
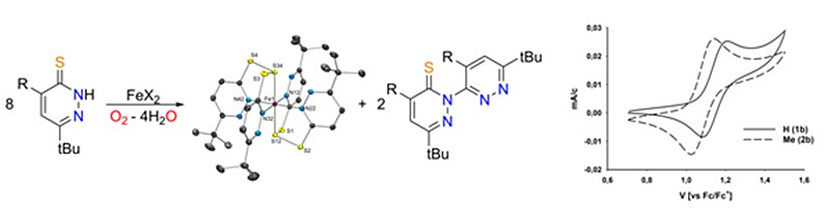
Thiopyridazine based metal complexes
In our group we focus on heterocyclic ligands with N, and S donor atoms. Special emphasis is put on differently substituted thiopyridazines and their selenium analogues. Due to their electron deficient nature, they exhibit unusual properties and reactivites. For example, C-N coupling reactions and high redox potentials are observed.
Fig. 1: Oxygen dependent synthesis and CV of an unusual irontrisulfide complex with concomitant formation of a C-N coupled dipyridazine.
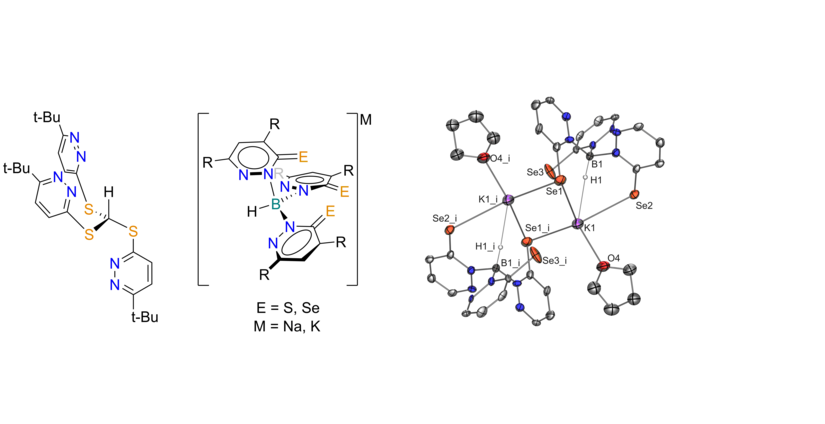
Scorpionate ligands
Scorpionates are tridentate, monoanionic ligands that enforce a facial coordination to a metal center. Therfore, they are frequently used for modeling tetrahedral active centers in enzymes. Our group uses pyridazines as backbones for soft scorpionate ligands. Additionally to their photo reactivity, they show interesting reactivity like the selective formation of metallaboratrane complexes. Part of our group focuses on the synthesis and the coordination properties of pyridazine based scorpionate ligands. We use boron, as well as carbon as central atom, in combination with different sulfur and selenium containing pyridazines.
Fig. 2: Carbon (left) and boron (right) based, pyridazine containing scorpionates and molecular structure of a [Se3] donor ligand.
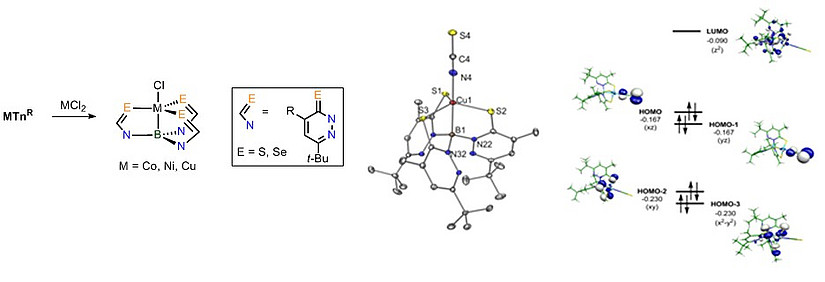
A special case: Boratrane complexes
Metallaboratrane complexes are tricyclic structures with a direct metal-boron bond. They belong to Z-type compounds, in which the metal center provides the electron pair for the formation of a dative bond. This results in unusual properties which we are investigating.
Fig. 3: Synthesis, crystal structure and calculated MO scheme of the frontier orbitals in a copper boratrane complex.